Avogadro'S Hypothesis Definition
Avogadro's Hypothesis Definition. Web medical definition of avogadro's law. Volume is a third way to measure the amount of matter, after item count and mass.
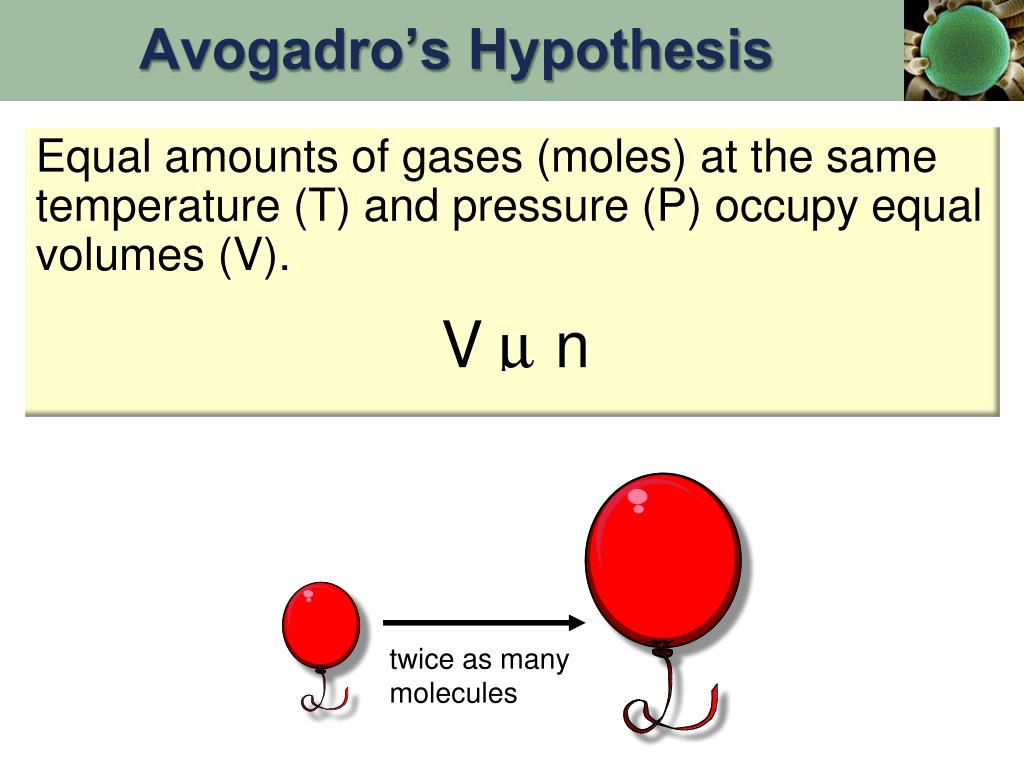
The law is a specific case of. | meaning, pronunciation, translations and examples Web noun the principle that equal volumes of all gases contain the same number of molecules at the same temperature and pressure collins english dictionary.
Web Avo·gadro's Hy·poth·e·sis Here Are All The Possible Meanings And Translations Of The Word Avogadro's Hypothesis.
Web this is known as avogadro’s hypothesis. Web applications of avogadro’s law: Web avogadro’s law is a gas law and is also referred to as avogadro’s hypothesis or avogadro’s principle.
Avogadro’s Number Is Approximately 6.022140857(74)×1023.
Avogadro's hypothesis states that two samples of gas of equal volume, at the same temperature and pressure, contain the same number of molecules. The law is a specific case of. A body of rules, regulations, and legal opinions of conduct and action that are made by controlling.
It States That Equal Volumes Of Any And All Gases At The Same Temperature And Pressure Contain The Same Number Of.
Web avogadro's hypothesis and molar volume. It is the number of. Web definitions of avogadro's hypothesis noun the principle that equal volumes of all gases (given the same temperature and pressure) contain equal numbers of molecules.
Avogadro’s Law Is Applied :
Web in 1811, italian physicist and mathematician amedeo avogadro published a hypothesis (also termed avogadro's law or principle) stating that the volume of a gas is directly. Avogadro's number, or avogadro's constant, is the number of particles found in one mole of a substance. Web avogadro's law (sometimes referred to as avogadro's hypothesis or avogadro's principle) is an experimental gas law relating the volume of a gas to the amount of.
Web The Number Of Units In One Mole Of Any Substance Is Called Avogadro's Number Or Avogadro's Constant.
Web noun the principle that equal volumes of all gases contain the same number of molecules at the same temperature and pressure collins english dictionary. It states that equal volume of all gases under similar condition of temperature and pressure. Volume is a third way to measure the amount of matter, after item count and mass.
Post a Comment for "Avogadro'S Hypothesis Definition"