Definition Of Equivalence Point In Chemistry
Definition Of Equivalence Point In Chemistry. 4 rows a point of equivalence in a titration refers to a point at which the added titrant is. Definition of equivalence 1a :
Examples of equivalence point in the following topics: The state or property of being equivalent. 4 rows a point of equivalence in a titration refers to a point at which the added titrant is.
If You Are Titrating An Acid Against A Base, The Half.
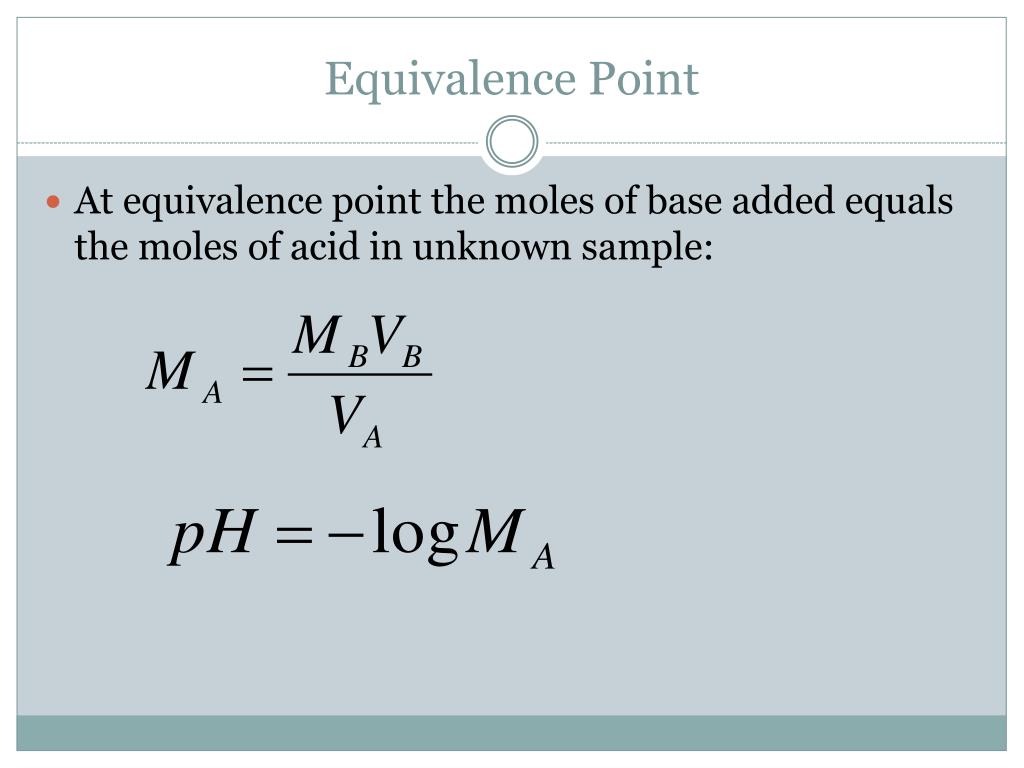
A point at which the moles of the titrant is equal to the moles of analyte in the solution titrant : The key difference between half equivalence point and equivalence point is that half equivalence point is the midpoint between the starting point and the equivalence point. The visible endpoint may or may not coincide with the equivalence.
Before You Begin The Titration, You Must Choose A Suitable Ph Indicator, Preferably One That Will Experience A Color.
After some volume of the base has been added, a point is reached which corresponds to the equal amount of acid and base in the mixture. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been mixed. Chemists typically record the results of an acid titration on a graph sheet where ph on the vertical axis and the volume of the base they are adding on the horizontal axis.
Examples Of Equivalence Point In The Following Topics:
The point in the titration process where the chemical reaction in the titration mixture ends is called the equivalence point. Equivalence point (noun) the point in a chemical reaction at which chemically equivalent quantities of acid and base have been mixed. Definition of equivalence 1a :
Definition Of Equivalence Point In Chemistry.
An equivalence point is a point at which the combined moles of two solutions equilibrate. The state or property of being equivalent. The point in the titration process.
The Point At Which Stoichiometric Amounts Of The Two Reactants Have Been Added Is Referred To As The Equivalence Point.
The relation holding between two statements if they are either both true or both false so that to affirm one and to. The moles of titrant (standard solution). It denotes that the titrant amount applied is chemically comparable to the sample in question.
Post a Comment for "Definition Of Equivalence Point In Chemistry"